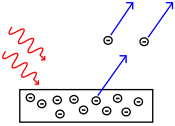
The photoelectric effect is the observation that many metals emit electrons when light shines upon them. Electrons emitted in this manner may be called photoelectrons.
According to classical electromagnetic theory, this effect can be attributed to the transfer of energy from the light to an electron in the metal. From this perspective, an alteration in either the amplitude or wavelength of light would induce changes in the rate of emission of electrons from the metal. .
The electrons are only dislodged by the photoelectric effect if light reaches or exceeds a threshold frequency, below which no electrons can be emitted from the metal regardless of the amplitude and temporal length of exposure of light. To make sense of the fact that light can eject electrons even if its intensity is low, Albert Einstein proposed that a beam of light is not a wave propagating through space, but rather a collection of discrete wave packets (photons), each with energy hf. This shed light on Max Planck's previous discovery of the Planck relation (E = hf) linking energy (E) and frequency (f) as arising from quantization of energy. The factor h is known as the Planck constant.
 of an ejected electron is given by
of an ejected electron is given by

where 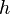 is the Planck constant and
is the Planck constant and 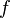 is the frequency of the incident photon. The term
is the frequency of the incident photon. The term 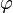 is the work function (sometimes denoted
is the work function (sometimes denoted  , or
, or  ),
which gives the minimum energy required to remove a delocalised
electron from the surface of the metal. The work function satisfies
),
which gives the minimum energy required to remove a delocalised
electron from the surface of the metal. The work function satisfies

where  is the threshold frequency for the metal. The maximum kinetic energy of an ejected electron is then
is the threshold frequency for the metal. The maximum kinetic energy of an ejected electron is then

Kinetic energy is positive, so we must have  for the photoelectric effect to occur.
for the photoelectric effect to occur.
According to classical electromagnetic theory, this effect can be attributed to the transfer of energy from the light to an electron in the metal. From this perspective, an alteration in either the amplitude or wavelength of light would induce changes in the rate of emission of electrons from the metal. .
The electrons are only dislodged by the photoelectric effect if light reaches or exceeds a threshold frequency, below which no electrons can be emitted from the metal regardless of the amplitude and temporal length of exposure of light. To make sense of the fact that light can eject electrons even if its intensity is low, Albert Einstein proposed that a beam of light is not a wave propagating through space, but rather a collection of discrete wave packets (photons), each with energy hf. This shed light on Max Planck's previous discovery of the Planck relation (E = hf) linking energy (E) and frequency (f) as arising from quantization of energy. The factor h is known as the Planck constant.
| Light–matter interaction |
|---|
 |
Mathematical derivation -
The maximum kinetic energy of an ejected electron is given by
of an ejected electron is given by
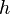 is the Planck constant and
is the Planck constant and 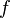 is the frequency of the incident photon. The term
is the frequency of the incident photon. The term 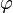 is the work function (sometimes denoted
is the work function (sometimes denoted  , or
, or  ),
which gives the minimum energy required to remove a delocalised
electron from the surface of the metal. The work function satisfies
),
which gives the minimum energy required to remove a delocalised
electron from the surface of the metal. The work function satisfies
 is the threshold frequency for the metal. The maximum kinetic energy of an ejected electron is then
is the threshold frequency for the metal. The maximum kinetic energy of an ejected electron is then
 for the photoelectric effect to occur.
for the photoelectric effect to occur.
0 comments:
Post a Comment