Definition - centred cubic crystals
A cubic crystal is a crystal system in which unit cell is a cube . There are three types of cubic crystals .
These are -
- Simple cubic crystals . (s.c )
- Face centred cubic or cubic closed packed crystals . (fcc )
- Body centred cubic crystals .( bcc )
Simple cubic crystals -
In this structure , the unit cell has atoms only at the corners of the cube . There are eight atoms lying at the corners and each atom contributes only 1/8 th of its effective part to a unit cell because it is shared by eight unit cells .
e.g - CsCl , NaCl , etc
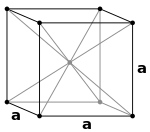
Face centred cubic or cubic closed packed crystals -
In this structure , atoms are at the corners of the cube as well as at the centre of each face .
It has a stacking sequence as ABC . This type of structure is closed packed because each atom is in contact with twelve atoms .
e.g - copper , aluminium , gold , lead ,silver , nickel and platinum etc .
Body centred cubic crystals -
In this structure , an atom is present at the centre of the body of unit cell in addition to the atoms lying at the corners .
e.g - sodium ,tungsten , potassium and molybdenum etc .
| Name | Primitive cubic | Body-centered cubic | Face-centered cubic |
|---|---|---|---|
| Pearson symbol | cP | cB | cF |
| Unit cell | 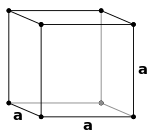 |
 |
 |
0 comments:
Post a Comment